A) sp2
B) d2sp3
C) sp
D) sp3
E) dsp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electron distributions among the molecular orbitals best describes the NO molecule? 
A) IV
B) V
C) III
D) I
E) II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the bond order of a bond increases, its bond energy ______ and its bond length ______.
A) decreases, increases
B) increases, increases
C) increases, decreases
D) decreases, decreases
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ethyl group (CH3CH2-) that is attached to a substituent that does not contain a hydrogen atom appears as what in a NMR spectrum?
A) a triplet and a quartet with relative intensities of 3 and 2, respectively
B) a triplet and a quartet with relative intensities of 2 and 3, respectively
C) a doublet and a triplet with relative intensities of 2 and 3, respectively
D) a doublet and a triplet with relative intensities of 3 and 2, respectively
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The fact that O2 is paramagnetic can be explained by
A) hybridization of atomic orbitals in O2.
B) the Lewis structure of O2.
C) the molecular-orbital diagram for O2.
D) resonance.
E) a violation of the octet rule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false?
A) The carbon-carbon bond in C22- is shorter than the one in CH3CH3.
B) The carbon-carbon bond in C22- is stronger than the one in CH3CH3.
C) C2 is diamagnetic.
D) C2 is paramagnetic.
E) Two of these statements are false.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the structure of glycine, the simplest amino acid: 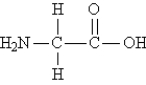 What is the total number of bonds in the molecule?
What is the total number of bonds in the molecule?
A) 10
B) 11
C) 7
D) 6
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to MO theory, which molecule(s) do not exist?
A) H ![]()
B) H ![]()
C) H ![]()
D) H ![]()
E) A, B, and D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are true of bond order? 1) If the number of bonding electrons is greater than the number of antibonding electrons in a given molecule, the molecule is predicted to be stable. 2) Bond order is the difference between the number of bonding electrons and the number of antibonding electrons, divided by 4. 3) If the bond order of a molecule is high, its bond strength is also high.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetracyanoethylene has the skeleton shown here: 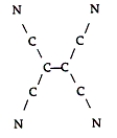 From its Lewis structure, determine the following.
-How many of the atoms are sp2 hybridized?
From its Lewis structure, determine the following.
-How many of the atoms are sp2 hybridized?
A) 2
B) 4
C) 10
D) 8
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the central atom in IBr4?
A) sp2
B) d2sp3
C) dsp3
D) sp
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are involved in pi bonding in benzene, C6H6?
A) 18
B) 6
C) 30
D) 3
E) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following homonuclear diatomic molecules, which is paramagnetic?
A) C2
B) B2
C) F2
D) N2
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the structure of glycine, the simplest amino acid: 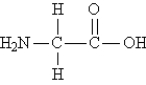 What is the total number of π bonds in the molecule?
What is the total number of π bonds in the molecule?
A) 0
B) 1
C) 2
D) 1/2
E) More information is needed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecule. (Lone pairs are not drawn in.) 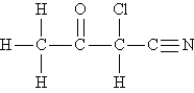 -What is the hybridization of the nitrogen atom?
-What is the hybridization of the nitrogen atom?
A) sp2
B) d2sp3
C) sp
D) sp3
E) dsp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) All antibonding MOs are higher in energy than the atomic orbitals of which they are composed.
B) Electrons are never found in an antibonding MO.
C) Antibonding MOs have electron density mainly outside the space between the two nuclei.
D) None of these statements is true.
E) Two of these statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecular-orbital energy-level diagrams for O2 and NO. Which of the following is true? I. Both molecules are paramagnetic. II. The bond strength of O2 is greater than the bond strength of NO. III. NO is an example of a homonuclear diatomic molecule. IV. The ionization energy of NO is smaller than the ionization energy of NO+.
A) I and II only
B) I, II, and IV
C) I and IV
D) II and III
E) I only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is paramagnetic?
A) N2
B) H2
C) C2
D) B2
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false?
A) Paramagnetic molecules are attracted toward a magnetic field.
B) Paramagnetism cannot be deduced from the Lewis structure of a molecule alone.
C) Atoms or molecules with an even number of electrons are diamagnetic.
D) N2 molecules are diamagnetic.
E) Atoms or molecules with an odd number of electrons are paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the I atom in the molecule IF5?
A) sp
B) sp3
C) dsp3
D) sp2
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 81
Related Exams