Correct Answer

verified
Correct Answer
verified
Essay
Consider the set of compounds,NH3,HF,and H2O.Rank these compounds in order of increasing acidity and discuss your rationale.
Correct Answer

verified
NH3 < H2O < HF When determining relative a...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The conjugate acid of H2O is
A) H3O-.
B) H3O.
C) H3O+.
D) HO-.
E) H2O+.
Correct Answer

verified
Correct Answer
verified
Essay
What is the product of the following Lewis acid-base reaction? 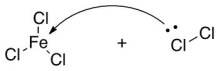
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the conjugate base of CH3NH2?
A) CH3NH3+
B) CH3NH-
C) NH4+
D) NH2-
Correct Answer

verified
Correct Answer
verified
Short Answer
Structure of Lorazepam,a widely known drug for short-term anxiety is shown below.Is the indicated lone pair localized or delocalized? 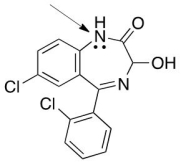
Correct Answer

verified
Correct Answer
verified
Essay
Draw a resonance contributor and the resonance hybrid for HOCO2-.
Correct Answer

verified
resonance ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Would you predict trifluoromethanesulfonic acid,CF3SO3H,to be a stronger or weaker acid than methanesulfonic acid,CH3SO3H? Explain your reasoning.
Correct Answer

verified
Trifluoromethanesulfonic acid is a stron...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Propanoic acid,CH3CH2COOH,has a pKa =4.9.Draw the structure of the conjugate base of propanoic acid and give the pH above which 90% of the compound will be in this conjugate base form.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
H-A is an acid with a pKa of 4.5.Which of the following statements about an aqueous solution of H-A is true?
A) At pH = 4.5,the solution contains much more H-A than.A-.
B) At pH = 4.5,the solution contains much more A- than H-A.
C) At pH- 3.5,the solution contains about 90% A- and 10% H-A.
D) At pH = 6.5,the solution contains about 80% A- and 20% H-A.
E) At pH = 5.5,the solution contains about 90% A- and 10% H-A.
Correct Answer

verified
Correct Answer
verified
Essay
Predict the direction of equilibrium in the following reaction.Explain your answer. 
Correct Answer

verified
Right Conjugate base on the ri...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is not a conjugate acid-base pair?
A) (H2O,HO-)
B) (H2O,H3O+)
C) (HSO4-,H2SO4)
D) (-OH,O2-)
E) (NO3-,NO2-)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the conjugate acid in the following acid base reaction? 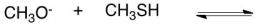
A) CH2O
B) CH3OH
C) CH3SH2+
D) CH3S-
E) H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a small amount of hexanoic acid [CH3(CH2) 4CO2H,pKa~4.8],is added to a separatory funnel which contains the organic solvent diethyl ether and water with a pH of 12.0,it is found mainly in the ________ phase as ________.
A) ether; CH3(CH2) 4CO2-
B) water; CH3(CH2) 4CO2-
C) ether; CH3(CH2) 4CO2H
D) water; CH3(CH2) 4CO2H
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product formed from the following acid-base reaction when ammonia functions as a base? The equilibrium lies far to the reactants. 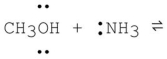
A) CH3O- + +NH4
B) CH2OH + +NH3
C) CH3OH2+ + -NH2
D) CH3NH2 + H2O
E) CH4 + NH2OH
Correct Answer

verified
Correct Answer
verified
Essay
2-Propanol is shown below.Draw the structure of its conjugate base. (CH3)2CHOH
Correct Answer

verified
Correct Answer
verified
Essay
If H2O has a pKa value of 15.7 and HF has a pKa value of 3.2,which is a stronger base,HO- or F-? Explain.
Correct Answer

verified
HO- is a stronger base than F- b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the compound with the highest pKa.
A) CH3NH2
B) CH3OH
C) CH3COOH
D) H2O
E) CH3NH3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) HF
B) H2O
C) : NH3
D) CH4
E) CH3OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3OH
B) CH3OH2+
C) H2N-
D) CH3NH2
E) CH3NH3+
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 43
Related Exams