Correct Answer

verified
Many metals emit electrons whe...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following statements is TRUE?
A) The emission spectrum of a particular element is always the same and can be used to identify the element.
B) Part of the Bohr model proposed that electrons in the hydrogen atom are located in "stationary states" or particular orbits around the nucleus.
C) The uncertainty principle states that we can never know both the exact location and speed of an electron.
D) An orbital is the volume in which we are most likely to find an electron.
E) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following quantum numbers describes the shape of an orbital?
A) principal quantum number
B) magnetic quantum number
C) spin quantum number
D) Schrödinger quantum number
E) angular momentum quantum number
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different values of ml are possible in the 5f sublevel?
A) 1
B) 7
C) 4
D) 5
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the orbital. 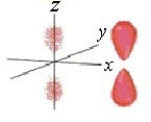
A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the correct values for a 4f orbital.
A) n = 3, l = 2, ml = 1
B) n = 2, l = 1, ml = 1
C) n = 1, l = 0, ml = 0
D) n = 2, l = 0, ml = 0
E) n = 4, l = 3, ml = -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For hydrogen,what is the wavelength of the photon emitted when an electron drops from a 4p orbital to a 2s orbital in a hydrogen atom? The Rydberg constant is 1.097 × 10-2 nm-1.
A) 656.3 nm
B) 486.2 nm
C) 364.6 nm
D) 2.057 × 10-3 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the color of a flame test for potassium.
A) violet
B) red
C) white
D) yellow
E) blue
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the correct values for a 3p orbital.
A) n = 3, l = 1, ml = 0
B) n = 2, l = 1, ml = 0
C) n = 1, l = 0, ml = 0
D) n = 2, l = 0, ml = 0
E) n = 4, l = -1, ml = +1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals are contained in the third principal level (n = 3) of a given atom?
A) 9
B) 1
C) 12
D) 6
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following transitions (in a hydrogen atom) represent emission of the longest wavelength photon?
A) n = 1 to n = 3
B) n = 3 to n = 2
C) n = 3 to n = 4
D) n = 5 to n = 2
E) n = 5 to n = 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which orbital below would an electron (on average) be closest to the nucleus?
A) 4f
B) 4s
C) 2s
D) 5d
E) 5p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following types of electromagnetic radiation in order of increasing wavelength. visible light x-rays microwaves
A) x-rays < microwaves < visible light
B) microwaves < visible light < x-rays
C) microwaves < x-rays < visible light
D) visible light < x-rays < microwaves
E) x-rays < visible light < microwaves
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sunburn is caused by overexposure to ________ radiation.
A) ultraviolet
B) gamma
C) microwave
D) x-ray
E) radio
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the correct values for a 1s orbital.
A) n = 3, l = 1, ml = +1
B) n = 2, l = 1, ml = 0
C) n = 1, l = 0, ml = 0
D) n = 2, l = 0, ml = -1
E) n = 4, l = 1, ml = -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different values of ml are possible in the 4d sublevel?
A) 2
B) 0
C) 3
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of d orbitals that are possible?
A) 2
B) 4
C) 8
D) 5
E) 11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following quantum numbers describes the size and energy of an orbital?
A) magnetic quantum number
B) principal quantum number
C) angular momentum quantum number
D) spin quantum number
E) Schrödinger quantum number
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electromagnetic radiation with a wavelength of 575 nm appears as yellow light to the human eye.The energy of one photon of this light is 3.46 × 10-19 J.Thus,a laser that emits 1.3 × 10-2 J of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
A) 2.7 × 10-17
B) 7.8 × 10-24
C) 2.2 × 1019
D) 3.8 × 1016
E) 6.5 × 1013
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the velocity of a marble (m = 8.11 g) with a wavelength of 3.46 × 10-33 m.
A) 42.3 m/s
B) 2.36 m/s
C) 23.6 m/s
D) 38.8 m/s
E) 52.9 m/s
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 134
Related Exams