A) 2.10 L
B) 2.80 L
C) 4.20 L
D) 8.40 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases has the lowest average speed at 25°C?
A) He
B) H2Se
C) PH3
D) F2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total volume of the mixture of hydrogen gas and oxygen gas can be obtained from the electrolysis of 130.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below? 2 H2O(l) → 2 H2(g) + O2(g)
A) 88.3 L
B) 176 L
C) 265 L
D) 529 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of 350.mL of gas at 25°C is decreased to 125 mL at constant pressure.What is the final temperature of the gas?
A) -167°C
B) 8.9°C
C) 70°C
D) 561°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of N2 are in a 200.0 mL container at 780 mm Hg and 135°C?
A) 3.50 × 1021 molecules
B) 3.69 × 1021 molecules
C) 1.06 × 1022 molecules
D) 1.12 × 1022 molecules
E) 3.30 × 1021 molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Near Cozumel,Mexico,a scuba diver goes down 60 ft to look at the sea creatures.As she descends,the air pressure increases.Which gas law is this an example of?
A) Avogadro's Law
B) Ideal Gas Law
C) Charles's Law
D) Boyle's Law
E) Dalton's Law
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert 2.00 atm to psi.
A) 59.8 psi
B) 875 psi
C) 29.4 psi
D) 42.0 psi
E) 1520 psi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a gas is proportional to number of moles of a gas is known as
A) Avogadro's Law.
B) Ideal Gas Law.
C) Charles's Law.
D) Boyle's Law.
E) Dalton's Law.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A balloon contains 0.76 mol O2,0.18 mol Ne,0.031 mol Xe and 0.026 mol CO2 at 749 mm Hg.What is the partial pressure of Ne?
A) 20 mm Hg
B) 23 mm Hg
C) 140 mm Hg
D) 570 mm Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the graph below,determine the gas that has the lowest density at STP. 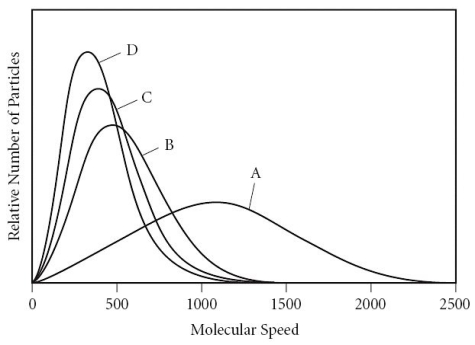
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of effusion of oxygen to an unknown gas is 1.49.What is the other gas?
A) Ar
B) HBr
C) SO2
D) Cl2
E) He
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume will a balloon occupy at 1.0 atm,if the balloon has a volume of 4.4 L at 2.2 atm?
A) 3.0 L
B) 1.50 L
C) 9.7 L
D) 6.6 L
E) 2.2 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atmospheric pressure is 715 mm Hg.What is the pressure in torr?
A) 715 torr
B) 28.1 torr
C) 13.5 torr
D) 29.5 torr
E) 760 torr
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the equation C2H6(g)+ O2(g)→ CO2(g)+ H2O(g)(not balanced),determine the number of liters of CO2 formed at STP when 120.0 grams of C2H6 is burned in excess oxygen gas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume (in mL) will a sample of F2 gas occupy in a syringe at 5.5 atm,if the F2 has a volume of 30.0 mL at 1.2 atm?
A) 138 mL
B) 30 mL
C) 4.0 mL
D) 6.5 mL
E) 1.2 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In Lhasa,Tibet,the elevation is 12,000 feet.The altimeter reading in an airplane is 19.00 in Hg.This pressure is equal to ________ atm.
A) 9.33
B) 483
C) 0.635
D) 1.57
E) 23.1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following samples has the greatest density at STP?
A) O2
B) Xe
C) CO2
D) SF6
E) All of these samples have the same density at STP.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 55.0-L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.39 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
A) 3.17 g
B) 8.20 g
C) 82.8 g
D) 1210 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the element or molecule that has the lowest diffusion rate.
A) Ne
B) CO
C) HCl
D) SO2
E) Xe
Correct Answer

verified
Correct Answer
verified
Short Answer
Define pressure.
Correct Answer

verified
Pressure is the force exerted ...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 41 - 60 of 182
Related Exams