Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: 2Cu+(aq) Cu(s) + Cu2+(aq) If the standard potentials of Cu2+ and Cu+ are +0.34 and +0.52 V,respectively,calculate the value of E for the given reaction.
A) +0.86 V
B) +0.70 V
C) +0.18 V
D) -0.18 V
E) -0.70 V
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following cell: Zn(s)|Zn2+(aq,0.10 M)m|Cu2+(aq,0.10 M)|Cu(s) At equilibrium,what is the concentration of Zn2+(aq)?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given: Cr(OH) 3(s) CrO42-(aq) ,basic solution. How many electrons appear in the balanced half-reaction?
A) 3
B) 6
C) 5
D) 7
E) 4
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell Pb(s) |Pb2+(aq) m Cu2+(aq) |Cu(s) Is +0.47 V.What is the standard potential of the Pb2+/Pb electrode?
A) -0.26 V
B) +0.81 V
C) -0.81V
D) -0.13 V
E) +0.13 V
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following metals,which metal would be suitable to provide an iron bridge with cathodic protection from corrosion?
A) Ni
B) Cu
C) Pb
D) Sn
E) None of the metals listed is suitable.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
For the reduction of Cu2+ by Zn, Go = -212 kJ/mol and Eo = +1.10 V.If the coefficients in the chemical equation for this reaction are multiplied by 2, Go = -424 kJ/mol.This means Eo = +2.20 V.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given: S2O42-(aq) SO32-(aq) ,basic solution. How many electrons appear in the balanced half-reaction?
A) 3
B) 6
C) 1
D) 4
E) 2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
In a working electrochemical cell (+ cell voltage),the electrons flow from the anode through the external circuit to the cathode.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the value of E for the following reaction? 2H+(aq,1.00 M)+ 2e-F H2(g,1.00 atm)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following metals would be suitable to provide cathodic protection from corrosion for an iron bridge?
A) Cu
B) Ni
C) Zn
D) Sn
E) Pb
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following diagram of a cell to answer questions 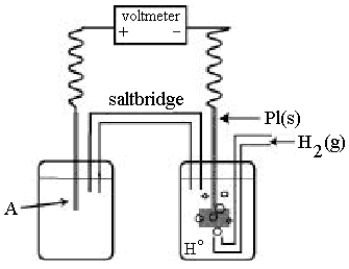 -Using the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,which half-reaction occurs in the left-hand cell compartment?
-Using the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,which half-reaction occurs in the left-hand cell compartment?
A) Ag(s) Ag+(aq) + e-
B) Ag+(aq) + e- Ag(s)
D) undefined
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell Ag(s) |AgCl(s) |Cl-(aq) m Cu2+(aq) |Cu(s) Is +0.12 V.What is the standard potential of the AgCl/Ag,Cl- electrode?
A) -0.46 V
B) -0.22 V
C) +0.24 V
D) +0.46 V
E) +0.22 V
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the following diagram of a cell to answer questions  -In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species will oxidize Cr2+ but not Mn2+?
A) Pb4+
B) O3 in acidic medium
C) Zn2+
D) Fe2+
E) V3+
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the determination of iron in vitamins,Fe2+ is titrated with permanganate,MnO4-,in acidic solution.The products of the reaction are Fe3+ and Mn2+.In the balanced equation,the number of electrons transferred is
A) 5
B) 1
C) 10
D) 7
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the following diagram of a cell to answer questions  -In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which electrode is negative?
-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which electrode is negative?
Correct Answer

verified
Correct Answer
verified
True/False
When KI(aq)is electrolyzed at a concentration of 1 M,the product at the anode is I2.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The half-reaction that occurs at cathode when 1 M AgNO3(aq)is electrolyzed is __________________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the cell diagram Pt|H2(g) ,H+(aq) m Cu2+(aq) |Cu(s) Which reaction occurs at the anode?
A) Cu(s) Cu2+(aq) + 2e-
B) 2H+(aq) + 2e- H2(g)
C) 2H+(aq) + Cu(s) H2(g) + Cu2+(aq)
D) Cu2+(aq) + 2e- Cu(s)
E) H2(g) 2H+(aq) + 2e-
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 94
Related Exams