A) C22+
B) N22+
C) B2
D) C22-
E) B22+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the electron-domain geometry of the central atom in SCl4 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle between the axial position and the equatorial position for a molecule with a trigonal bipyramidal geometry.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for N2H2.What is the hybridization on the N atoms?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 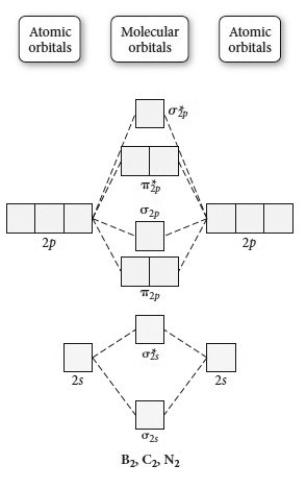
A) B22+
B) B22-
C) N22+
D) C22-
E) B2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw a molecular orbital diagram and use it to determine which of the following is most stable.
A) C22+
B) N22+
C) B2
D) C22-
E) B22+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules contain at least one pi bond? C2H6 Cl2CO C2Cl4 SeS3
A) 0
B) 1
C) 3
D) 4
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw a molecular orbital diagram and use it to determine which of the following is paramagnetic.
A) O22-
B) Ne22+
C) O22+
D) F22+
E) None of the above is paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of increasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. HCN H2O H3O+
A) H3O+ < HCN < H2O
B) H3O+ < H2O < HCN
C) HCN < H3O+ < H2O
D) H2O < HCN < H3O+
E) H2O < H3O+ < HCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle between two equatorial positions for a molecule with a trigonal bipyramidal geometry.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of XeF2.
A) eg = trigonal bipyramidal,mg = bent
B) eg = linear,mg = linear
C) eg = tetrahedral,mg = linear
D) eg = trigonal bipyramidal,mg = linear
E) eg = tetrahedral,mg = bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with T-shape molecular geometry.
A) <90°
B) 90°
C) >90°
D) <120°
E) 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybrid orbital set used by the central atom in SCl4 is ________.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry for the molecule XeF4.
A) Linear
B) Trigonal planar
C) Tetrahedral
D) Trigonal bipyramidal
E) Octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with a linear shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the electron-domain geometry of the central atom in BrF4- is ________.
A) tetrahedral
B) trigonal bipyramidal
C) trigonal planar
D) octahedral
E) linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of CO2.
A) eg = tetrahedral,mg = tetrahedral
B) eg = linear,mg = trigonal planar
C) eg = trigonal planar,mg = bent
D) eg = linear,mg = linear
E) eg = trigonal planar,mg = trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule PCl5.
A) Linear
B) Seesaw
C) Square pyramidal
D) Trigonal bipyramidal
E) Square planar
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 158
Related Exams