A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has polar bonds but is a nonpolar molecule
A) PCl3
B) NCl3
C) BF3
D) HF
E) OCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An sp2 hybridized central carbon atom with no lone pairs of electrons has what type of bonding
A) 1 and 2 bonds
B) 1 and 3 bonds
C) 2 and 2 bonds
D) 3 and 2 bonds
E) 0 and 4 bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the molecular geometry of CBr4.
A) 0 lone pairs, square planar
B) 0 lone pairs, tetahedral
C) 1 lone pair, square pyramidal
D) 1 lone pair, trigonal bipyramidal
E) 2 lone pairs, square planar
Correct Answer

verified
Correct Answer
verified
True/False
A species with a bond order of 1/2 may be stable.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the geometry and polarity of the CS2 molecule.
A) linear, polar
B) linear, nonpolar
C) tetrahedral, nonpolar
D) bent, nonpolar
E) bent, polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the molecular geometry and polarity of the SO2 molecule.
A) linear, polar
B) linear, nonpolar
C) bent, polar
D) bent, nonpolar
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances is/are bent (i) H2S (ii) . CO2 (iii) ClNO (iv) NH2- (v) O3
A) only (iii)
B) (i) and (v)
C) (i) , (iii) , and (v)
D) (i) , (ii) , (iii) , and (v)
E) (i) , (iii) , (iv) , and (v)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the molecular geometry of XeF2.
A) 0 lone pairs, linear
B) 1 lone pair, bent
C) 2 lone pairs, bent
D) 3 lone pairs, bent
E) 3 lone pairs, linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the geometry around the central atom in SO42-.
A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The F -Cl -F bond angles in ClF3 are expected to be approximately
A) 90 only.
B) 109.5 only.
C) 120 only.
D) 180 only.
E) 90 and 180 .
Correct Answer

verified
Correct Answer
verified
True/False
CO2 is nonpolar, but OCS is polar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using periodic trends, arrange the following molecules in order of increasing dipole moment: NH3, PH3, AsH3.
A) AsH3 < PH3 < NH3
B) PH3 < NH3 < AsH3
C) NH3 < AsH3 < PH3
D) AsH3 < NH3 < PH3
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond angles are present in a molecule with an octahedral geometry
A) 45
B) 90
C) 120
D) depends on the type of atoms in the octehedron
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An sp hybridized central carbon atom with no lone pairs of electrons has what type of bonding
A) 1 and 2 bonds
B) 1 and 3 bonds
C) 2 and 2 bonds
D) 3 and 2 bonds
E) 0 and 4 bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has the largest dipole moment (i.e., is the most polar)
A) H2
B) H2O
C) H2S
D) H2Se
E) CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below 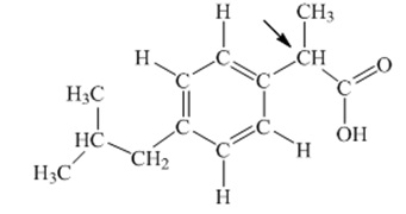
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
True/False
A molecule with polar bonds must be a polar molecule in all cases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sp3d hybridized central atom has what angles between its hybrid orbitals
A) 120 only
B) 120 and 109.5
C) 109.5 only
D) 120 and 90
E) < 90 only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the species O2-, O2, and O2+. Which of these species will be paramagnetic
A) O2 and O2-
B) O2+ and O2
C) O2+ and O2-
D) only O2
E) all three are paramagnetic
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 146
Related Exams