A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with trigonal planar electron geometry and bent molecular geometry.
A) <90°
B) 90°
C) <120°
D) <109.5°
E) 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) , molecular geometry (mg) , and polarity of SF6.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal, nonpolar
B) eg = tetrahedral, mg = tetrahedral, polar
C) eg = trigonal bipyramidal, mg = seesaw, polar
D) eg = octahedral, mg = trigonal bipyramidal, nonpolar
E) eg = octahedral, mg = octahedral, nonpolar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule SBr2.
A) Trigonal pyramidal
B) Trigonal planar
C) Tetrahedral
D) T-shaped
E) Bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, the electron-domain geometry of the central atom in SF4 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for the molecule C3H4. How many sigma and pi bonds does it contain?
A) 7 sigma, 1 pi
B) 8 sigma, 0 pi
C) 6 sigma, 2 pi
D) 10 sigma, 0 pi
E) 8 sigma, 2 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of XeF4.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with trigonal bipyramidal electron geometry and seesaw molecular geometry.
A) <120° (equatorial) , 90° (axial)
B) 120° (equatorial) , <90° (axial)
C) <90° (equatorial) , <90° (axial)
D) 120° (equatorial) , 90° (axial)
E) <120�° (equatorial) , <90° (axial)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, the molecular geometry of the central atom in XeF2 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The orbital hybridization on the carbon atoms in C2H2 is ________.
A) sp
B) sp2
C) sp3
D) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In molecular orbital theory the acronym LCAO stands for
A) linear combination of atomic orbitals.
B) lowest combined atomic orbitals.
C) least consumed advanced orientation.
D) linearly created available orbital.
E) It has no meaning.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is least stable. 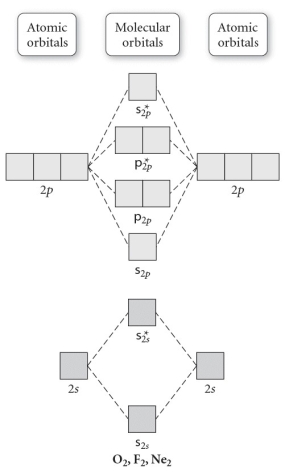
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F21⁻
Correct Answer

verified
Correct Answer
verified
Essay
Explain why oil and water do not mix.
Correct Answer

verified
Water molecules are ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the approximate bond angle for a molecule with octahedral electron geometry and square planar molecular geometry.
A) 90°
B) 180°
C) >120°
D) <90°
E) <120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for OF2. What is the hybridization on the O atom?
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NCl3?
A) T-shaped
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? XeO2 SiCl2Br2 C2Br2 SeCl6
A) 1
B) 4
C) 2
D) 3
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 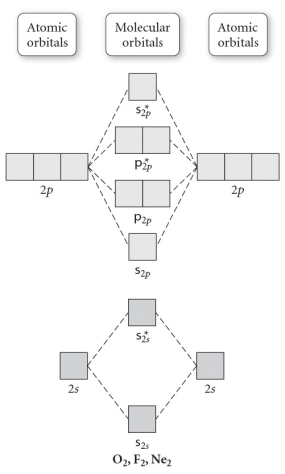
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -sp3d2
A) tetrahedral
B) nonpolar, but contains a polar covalent bond
C) sp hybridized central atom
D) trigonal bipyramidal
E) trigonal planar
F) polar
G) linear
H) seesaw molecular geometry
I) octahedral
J) octahedral electron geometry
K) polar, but contains no polar bonds
L) sp2 hybridized central atom
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule containing a central atom with sp hybridization has a ________ electron geometry.
A) linear
B) trigonal bipyramidal
C) trigonal planar
D) tetrahedral
E) bent
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 168
Related Exams