A) Avogadro's law
B) Ideal gas law
C) Charles's law
D) Boyle's law
E) Dalton's law
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert 717.28 mmHg to bar.
A) 1.046 bar
B) 0.9438 bar
C) 1.060 bar
D) 0.9563 bar
E) 0.8971 bar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.75 L container filled with CO2 gas at 25 °C and 2.25 bar springs a leak. When the container is resealed, the pressure is 1.85 bar and the temperature is 10 °C. How many moles of gas were lost?
A) 0.0335 mol
B) 0.728 mol
C) 0.882 mol
D) 3.39 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What pressure will 14.0 g of CO exert in a 3.5 L container at 75 °C?
A) 4.1 bar
B) 5.0 bar
C) 6.4 bar
D) 1.1 bar
E) 2.3 bar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following samples will have the greatest volume at STP?
A) 22 g CO
B) 22 g He
C) 22 g O2
D) 22 g Cl2
E) All of these samples would have the same volume at STP.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the temperature and pressure at STP.
A) 0° C and 1.00 bar
B) 0 K and 1.00 bar
C) 25° C and 30.00 in Hg
D) 300 K and 1 Torr Hg
E) 0° C and 1 mmHg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas bottle contains 0.250 mol of gas at 0.973 bar pressure. If the final pressure is 1.165 bar, how many moles of gas were added to the bottle?
A) 0.0262 mol
B) 0.0493 mol
C) 0.276 mol
D) 0.299 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -Boyle's law
A) measures blood pressure
B) measures pressure of a gas
C) V1/n1 = V2/n2
D) PV = nRT
E) P1V1 = P2V2
F) measures atmospheric pressure
G) V1/T1 = V2/T2
H) PT = PA + PB + PC ...
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert 1.25 atm to bar.
A) 1.23 bar
B) 0.975 bar
C) 1.27 bar
D) 0.7874 bar
E) 1.25 bar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert 3.1473 atm to bar.
A) 4.976 bar
B) 3.189 bar
C) 2.577 bar
D) 4.112 bar
E) 3.875 bar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the graph below, determine the gas that has the lowest density at STP. 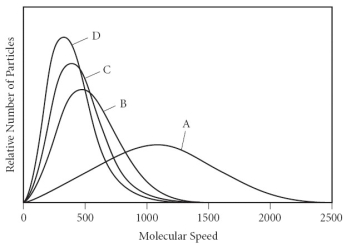
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the definition of diffusion?
A) gas molecules mix equally
B) gas molecules spread out in a concentration gradient
C) gas molecules escape from a container into a vacuum through a small hole
D) average distance between collisions
E) gas molecules mix unequally
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 0.24 mol of He were added to a balloon, how large would the balloon grow? Atmospheric pressure is 0.9871 bar and the ambient temperature is 23.2 °C.
A) 5.99 L
B) 6.54 L
C) 3.73 L
D) 6.49 L
E) 7.28 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the density of CO2 gas at 278 K and 1.00 bar.
A) 1.14 g L-1
B) 2.37 g L-1
C) 1.90 g L-1
D) 3.08 g L-1
E) 2.11 g L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the temperature of NO2 gas if the average speed (actually the root mean square speed) of the molecules is 750 m s-1?
A) 1.38 K
B) 1.04 × 103 K
C) 1.38 × 103 K
D) 1.04 × 106 K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the density of O3 gas at 341 K and 2.14 bar.
A) 3.62 g L-1
B) 2.91 g L-1
C) 0.321 g L-1
D) 4.82 g L-1
E) 3.17 g L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 0.8386 bar. Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 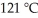 while maintaining the pressure at 0.8386 bar.
while maintaining the pressure at 0.8386 bar.
A) 10.9 L
B) 13.2 L
C) 2.07 L
D) 7.56 L
E) 48.4 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following samples will have the greatest average speed at 355 K?
A) Ne
B) C2H4
C) Cl2
D) CH4
E) All of these samples will have the same average speed at the same temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of XeF6 are formed from 12.9 L of F2 (at 298 K and 2.635 bar) according to the following reaction? Assume that there is excess Xe. Xe(g) + 3F2(g) → XeF6(g)
A) 1.21 × 1023 molecules XeF6
B) 8.25 × 1023 molecules XeF6
C) 2.75 × 1023 molecules XeF6
D) 7.29 × 1023 molecules XeF6
E) 1.37 × 1023 molecules XeF6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert 29.592 inches Hg to bar.
A) 0.9889 bar
B) 1.011 bar
C) 1.002 bar
D) 1.023 bar
E) 0.9980 bar
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 154
Related Exams