A) )
B) ( )
C) ( *)
D) ( *)
E) S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below regarding bonding theories is FALSE?
A) Lewis structures are usually adequate when focusing on row 2 atoms and their bonding patterns.
B) The magnetic and spectroscopic properties of molecules are best described using Lewis structures.
C) Valence bond theory is useful in understanding the shapes of molecules.
D) VSEPR theory allows us to predict molecular geometry.
E) MO theory explains magnetic and spectroscopic properties of molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound below has the largest dipole moment?
A) CF2Cl2
B) CH2Cl2
C) CH2F2
D) CF4
E) CH2Br2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound below has a trigonal bipyramidal molecular geometry?
A) ICl3
B) BrF5
C) PCl5
D) PH3
E) SiF4
Correct Answer

verified
Correct Answer
verified
Short Answer
Draw the Lewis structure with the lowest formal charges for XeOF2.What are the electronic geometry and molecular geometry?
Correct Answer

verified
 trigonal ...
trigonal ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the hybridization of the central iodine atom in I3- ?
A) sp
B) sp2
C) sp3
D) sp3d2
E) sp3d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The local molecular geometry and the hybridization around each carbon atom in benzene (C6H6 with a hexagonal ring structure) is _______
A) square planar and sp.
B) trigonal planar and sp.
C) tetrahedral and sp3.
D) trigonal planar and sp2.
E) T-shaped and sp2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of carbon in C2H2?
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion below has a central atom with the same hybridization as PCl5?
A) IF6+
B) SiF4
C) BrF5
D) SbF5
E) XeF4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of Xe in XeOF4, and what is the molecular geometry?
A) sp3d, T-shaped
B) sp3d, seesaw
C) sp3d2, square pyramidal
D) sp3d2, square planar
E) sp3d2, octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SF4?
A) tetrahedral
B) square pyramidal
C) seesaw
D) square planar
E) T-shaped
Correct Answer

verified
Correct Answer
verified
Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below.Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3.Identify the bond angles around C1, C2, and O3. 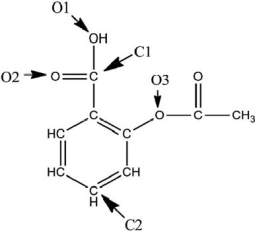
Correct Answer

verified
O1 is sp3 hybridized, O2...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which molecule or ion below has a trigonal pyramidal molecular geometry?
A) KrF4
B) COCl2
C) SeO32-
D) NH4 +
E) O3
Correct Answer

verified
Correct Answer
verified
Essay
ICl3 and SO3 have one central atom.Do they share the same molecular geometry? What is the hybridization of the central atom in each?
Correct Answer

verified
ICl3 has five electron groups a...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Explain the phenomenon of light absorption and emission from molecules.
Correct Answer

verified
Electrons in molecules can mov...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What are the ideal bond angles around carbon in CH2O?
A) 180
B) 90
C) 120
D) 109.5
E) 60
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule below is T-shaped molecular geometry?
A) NCl3
B) PCl3
C) SOCl2
D) ICl3
E) COCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion below paramagnetic?
A) Li2
B) N22-
C) Be2
D) O22-
E) F2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond angle is the smallest?
A) O - S - O in SO2
B) O - S - O in SO3
C) O - S - O in SO42-
D) F - S - F in SF6
E) Cl - S - Cl in SOCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the bond order of the OF molecule.
A) 2
B) 2.5
C) 3
D) 1.5
E) 1
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 141
Related Exams