A) H3O+, H2O
B) HNO2, NO2¯
C) H2SO4, HSO4¯
D) H2S, S2¯
E) NH3, NH2¯
Correct Answer

verified
Correct Answer
verified
True/False
All Lewis acids contain at least one proton.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of Ka for the methylammonium ion, CH3NH3+? Kb(CH3NH2) = 4.4 *10¯4
A) 4.4 * 10¯4
B) 4.8 * 10¯6
C) 4.4 * 10¯10
D) 2.3 * 10¯11
E) 4.4 * 10¯18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.20 M HCl solution?
A) < 0
B) 0.70
C) 1.61
D) 12.39
E) 13.30
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance Ca(OH) 2 is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.25 M solution of the weak acid HA is 9.2% dissociated. What is the pH of the solution?
A) 0.64
B) 0.94
C) 1.13
D) 2.16
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
True/False
The ammonium ion, NH4+, is a weak acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following aqueous liquids is the most acidic?
A) 0.1 M Al(NO3) 3, Ka = 1 *10¯5
B) 0.1 M Be(NO3) 2, Ka = 4 *10¯6
C) 0.1 M Pb(NO3) 2, Ka = 3 *10¯8
D) 0.1 M Ni(NO3) 2, Ka = 1 * 10¯10
E) pure water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by adding 0.10 mol of potassium chloride, KCl, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) One needs to know the temperature before any of the above predictions can be made.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.050 M HBr solution?
A) 0.89
B) 1.12
C) 1.30
D) 3.00
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
True/False
In order to be a Brønsted-Lowry base, a species must contain a proton.
Correct Answer

verified
Correct Answer
verified
True/False
The chloride ion, Cl¯, is a typical Lewis acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of Kb for the cyanide anion, CN¯? Ka(HCN) = 6.2 * 10¯10
A) 1.6 * 10¯4
B) 1.6 * 10¯5
C) 3.8 *10¯4
D) 3.8 * 10¯5
E) 6.2 * 104
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution is prepared by dissolving the salt formed by the neutralization of a weak acid by a weak base. Which statement about the solution is correct?
A) The solution is strongly basic.
B) The solution is weakly basic.
C) The solution is neutral.
D) The solution is acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aqueous solutions of phosphoric acid and sodium nitrite are combined, and the following equilibrium is established. 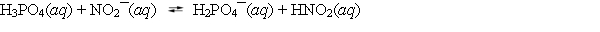 The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
A) Phosphoric acid is a weaker acid than nitrous acid.
B) Nitrous acid is a weaker acid than water.
C) The nitrite anion is a weaker base than the dihydrogen phosphate anion.
D) The dihydrogen phosphate anion is a stronger acid than nitrous acid.
E) Phosphoric acid is a stronger acid than nitrous acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a strong acid?
A) H2CO3
B) H2SO3
C) H2SO4
D) H3PO4
E) CH3COOH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lactic acid has a pKa of 3.08. What is the approximate degree of dissociation of a 0.35 M solution of lactic acid?
A) 1.1%
B) 2.2%
C) 4.8%
D) 14%
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest base?
A) CH3NH2
B) NaNO3
C) B(OH) 3
D) Al(OH) 3
E) LiOH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance HOBr is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances will give an aqueous solution of pH closest to 7?
A) KNO3
B) CO2
C) NH4I
D) NH3
E) CH3NH2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 106
Related Exams