A) CH3I > CH3Br > CH3Cl > CH3F
B) CH3F > CH3Cl > CH3Br > CH3I
C) CH3Cl > CH3F > CH3Br > CH3I
D) CH3Cl > CH3I > CH3F > CH3Cl
Correct Answer

verified
Correct Answer
verified
Short Answer
The major products of the reaction would be formed from the radical intermediate numbered _____ as shown below.
1.  2. 11ee515b_68f3_8e8d_b56e_e79049de47c8_TB7078_11
2. 11ee515b_68f3_8e8d_b56e_e79049de47c8_TB7078_11
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 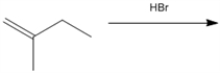
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the characteristic of a radical chain termination step?
A) Radicals are formed.
B) Substitution products are formed.
C) A radical reacts with a molecule to give a new radical and a new molecule.
D) Two radicals combine to give a molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding the halogenation of alkanes upon treatment with halogen and light?
A) Bromination is more selective for 3° positions than chlorination.
B) The reaction proceeds via a radical intermediate.
C) The reaction proceeds via a chain reaction.
D) This is a useful process for the formation of fluorides, chlorides, bromides and iodides.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of orbitals overlap to provide stability to the tert-butyl radical by hyperconjugation?
A) 3° C 2p atomic orbital + 3° C sp2 atomic orbital
B) 3° C 2p atomic orbital + methyl C−H σ molecular orbital
C) 3° C sp2 atomic orbital + methyl C−H σ molecular orbital
D) 3° C 2p atomic orbital + methyl C 2s atomic orbital
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the IUPAC name of the following compound (shown as a Fischer projection). 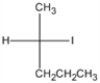
Correct Answer

verified
Correct Answer
verified
Essay
What is the structure of all of the monobrominated products, including regioismers and stereoisomers, obtained from the following reaction? 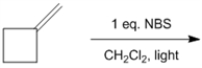
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about the allyl radical
A) The carbon-carbon bond lengths are identical.
B) The unpaired electron density is shared between carbons 1 and 2.
C) It undergoes reaction with bromine to give a single product.
D) It is formed by abstraction of a hydrogen atom from the methyl group of propene.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of reactive intermediate is formed upon irradiation of a solution of toluene (Ph-CH3) and bromine?
A) benzylic carbocation
B) benzylic carbanion
C) benzylic radical
D) cyclic bromonium ion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the characteristic of a radical chain propagation step?
A) Radicals are formed.
B) Byproducts are formed.
C) A radical reacts with a molecule to give a new radical and a new molecule.
D) Two radicals combine to give a molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the characteristic of a radical chain initiation step?
A) Radicals are formed.
B) Substitution products are formed.
C) A radical reacts with a molecule to give a new radical and a new molecule.
D) Two radicals combine to give a molecule.
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of possible monobromination products of cyclopentane is______.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkenes undergoes allylic bromination to form a single monobrominated product? 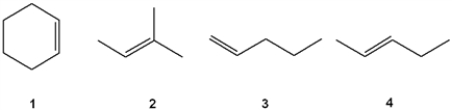
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the reaction below to answer the following question(s). -The number of allylic positions in compound A is ______.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 75 of 75
Related Exams