A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of XeO2F2 as predicted by the VSEPR theory? 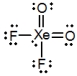
A) square planar
B) tetrahedral
C) square pyramidal
D) see-saw
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following is the best Lewis structure a resonance structure? (central atoms are bold)
A) CO2
B) ClO3-
C) COCl2
D) NO2+
E) HCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX5E will have a ______ molecular shape.
A) tetrahedral
B) trigonal bipyramidal
C) square pyramidal
D) octahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX4E2 will have a _____ molecular shape.
A) tetrahedral
B) square pyramidal
C) square planar
D) octahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best Lewis structure for ClCN.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of SiF62- as predicted by the VSEPR theory? 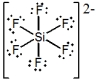
A) trigonal bipyramidal
B) hexagonal
C) tetrahedral
D) see-saw
E) octahedral
Correct Answer

verified
Correct Answer
verified
True/False
Bond angles of 180° only occur around atoms which display linear molecular geometry.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the nitrate ion (NO3-) , nitrogen and oxygen are held together by
A) ionic interactions.
B) covalent bonds.
C) dative bonds.
D) electronegativity.
E) network bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the hemoglobin molecule that carries oxygen molecules in blood, iron has 6 single bonds to surrounding atoms. What is the most likely geometry of these bonds to iron?
A) planar
B) linear
C) tetrahedral
D) octahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List all possible molecular geometries (shapes) for a nonpolar molecule with the formula AX4.
A) tetrahedral
B) see-saw
C) square planar
D) either tetrahedral or square planar
E) any of the above shapes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electron pairs are shared between the carbon atoms in C2H4?
A) 5
B) 4
C) 3
D) 2
E) 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the electron group arrangement around iodine, the central atom in the ion IF2-.
A) octahedral
B) trigonal bipyramidal
C) tetrahedral
D) trigonal planar
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charges on Cl and O in the structure shown for the ClO- ion are, respectively 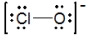
A) 0 and -1
B) -1 and 0
C) 1 and -2
D) -2 and 1
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the COCl2 molecule, carbon is the central atom. Based on the best Lewis structure for COCl2, what is the formal charge on carbon?
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the Lewis structure for XeO2F2 that correctly minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following species is the best Lewis structure a resonance structure?
A) NH3
B) CO2
C) SF6
D) O2
E) CO32-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the actual bond angles in SF3+ using the VSEPR theory.
A) more than 120°
B) exactly 120°
C) between 109° and 120°
D) between 90° and 109°
E) less than 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of NOCl as predicted by the VSEPR theory? 
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of NH2Cl as predicted by the VSEPR theory?
A) trigonal pyramidal
B) tetrahedral
C) T-shaped
D) see-saw
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 98
Related Exams