A) Cycloaddition reaction
B) Electrocyclic reaction
C) Electrophilic reaction
D) Sigmatropic reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many p molecular orbitals are present in 1,3,5-hexatriene?
A) 3
B) 4
C) 5
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a correct designation for a sigmatropic rearrangement?
A) [1,3]
B) [1,5]
C) [3,3]
D) [3,1]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following oxy-Cope rearrangement? 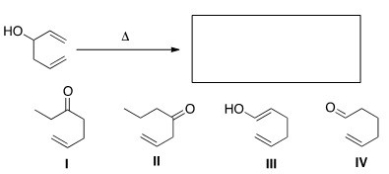
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the excited state LUMO for hexa-1,3,5-triene?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about [2+2] cycloaddition reactions between two alkenes is true?
A) The reaction is initiated by heat.
B) The reaction is initiated by light.
C) The product is a cyclopentane derivative.
D) Each reactant contains two s electrons that participate in the formation of new bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the Diels-Alder reaction called a thermal [4+2] cycloaddition?
A) Because the reaction is initiated by heat; the diene has four p electrons and the dienophile has two p electrons.
B) Because the reaction is initiated by light; the diene has four p electrons and the dienophile has two p electrons.
C) Because the reaction is initiated by heat; the dienophile has four p electrons and the diene has two p electrons.
D) Because the reaction is initiated by light; the dienophile has four p electrons and the diene has two p electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about pericyclic reactions is true?
A) Pericyclic reactions occur by way of ionic intermediates.
B) Pericyclic reactions occur by way of radical intermediates.
C) Pericyclic reactions involve multiple steps.
D) Reactive intermediates are not formed in pericyclic reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about pericyclic reactions is true?
A) In pericyclic reactions,bonds are broken and formed in multiple steps.
B) In pericyclic reactions,all bonds are broken and formed in a single step.
C) One intermediate has been identified in pericyclic reactions.
D) The transition state in a pericyclic reaction is acyclic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many p molecular orbitals are present in 1,3,5,7,9-decapentaene?
A) 4
B) 5
C) 10
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the ground state LUMO for buta-1,3-diene?
A) 0
B) 2
C) 4
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the ground state LUMO for hexa-1,3,5-triene?
A) 0
B) 2
C) 4
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the excited state HOMO for hexa-1,3,5-triene?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of sigmatropic rearrangement is illustrated below? ![What type of sigmatropic rearrangement is illustrated below? A) [1,3] B) [1,4] C) [3,3] D) [1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f776_79bc_a9af_5f6f4943ae16_TB7662_00.jpg)
A) [1,3]
B) [1,4]
C) [3,3]
D) [1,5]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about orbital symmetry and cycloaddition reactions is true?
A) Thermal cycloadditions involving an even number of p bonds proceed by a suprafacial pathway.
B) Thermal cycloadditions involving an odd number of p bonds proceed by an antarafacial pathway.
C) Photochemical cycloadditions involving an even number of p bonds proceed by an antarafacial pathway.
D) Photochemical cycloadditions involving an even number of p bonds proceed by a suprafacial pathway.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about electrocyclic reactions is true?
A) An electrocyclic reaction is generally irreversible.
B) Generally,an acyclic triene is favored over a six-membered ring at equilibrium.
C) Generally,a four-membered ring is favored over an acyclic diene at equilibrium.
D) An electrocyclic reaction is generally reversible.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of cycloaddition reaction is shown in the following equation? ![What type of cycloaddition reaction is shown in the following equation? A) [2+2] B) [4+2] C) [4+4] D) [0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f775_684b_a9af_2b3c0e539d3d_TB7662_00.jpg)
A) [2+2]
B) [4+2]
C) [4+4]
D) [0+2]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct classification of the following reaction? 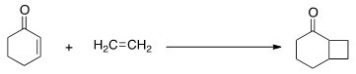
A) Cycloaddition reaction
B) Electrocyclic reaction
C) Electrophilic reaction
D) Sigmatropic reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major organic product(s) of the following electrocyclic reaction. 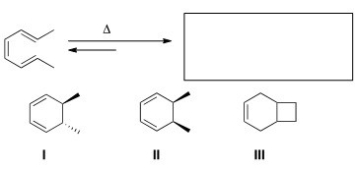
A) Only I
B) Only II
C) Only III
D) Only I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Cope rearrangement is not true?
A) The Cope rearrangement involves the rearrangement of a 1,5-diene to an isomeric 1,5-diene.
B) The Cope rearrangement takes place readily in a suprafacial pathway under photochemical conditions.
C) The Cope rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D) The Cope rearrangement takes place readily in a suprafacial pathway,when heated.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 62
Related Exams