A) The spin-spin splitting for the protons on the carbon next to the halogen will be different.
B) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted downfield relative to the protons on the carbon attached to the bromine atom.
C) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted upfield relative to the protons on the carbon attached to the bromine atom.
D) The integration of the protons on the carbon next to the halogen will be different.
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the proton-coupled 13C-NMR spectrum of the following molecule,how many doublets would be observed? 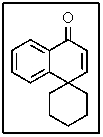
A) 2
B) 4
C) 6
D) 8
E) None of the above.
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The NMR peak intensities for a first-order quartet are:
A) 1:2:2:1
B) 1:3:3:1
C) 1:4:4:1
D) 1:1:1:1
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following 1H-NMR was most likely obtained from which of the compounds listed below? 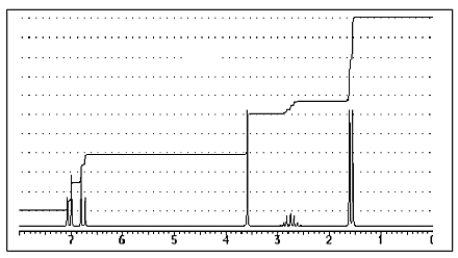
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The natural abundance of 13C is
A) 2.1%.
B) 10%.
C) 1.5%.
D) 1.1%.
E) 16%.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following is not true of 13C NMR?
A) The sensitivity of 13C NMR is much less than that of proton NMR.
B) By simultaneously decoupling the proton region,13C peaks appear as singlets.
C) (13C chemical shifts cover a much larger range than do 1H chemical shifts.)
D) The 13C NMR spectrum can show more peaks than there are carbons in the molecular formula.
E) The 13C NMR spectrum will show one peak for each type of carbon in a molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures C5H12O would give the NMR spectrum shown? 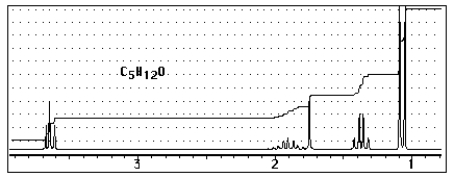
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures will give three signals (not counting TMS) in the proton decoupled 13C NMR?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The proton NMR spectrum of a carboxylic acid,C13H18O2,commonly sold as an over-the-counter headache remedy,is shown below.Note: the CO2H proton is not shown,and the multiplicity of the peaks are noted (d = doublet,q = quartet,m = multiplet) .Which structure is that of this pharmaceutical? 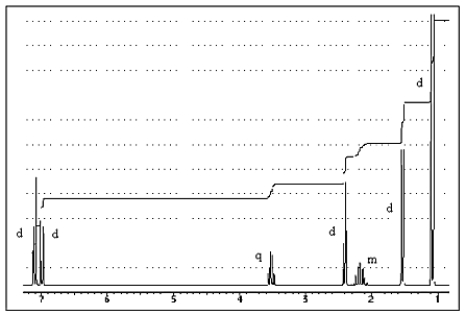
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In nuclear magnetic resonance,stronger magnetic fields
A) give a higher sensitivity spectrum than do lower magnetic fields.
B) give different chemical shifts (in ppm) than would weaker magnets.
C) give better separation between peaks in the spectrum (in Hz) than would weaker magnets.
D) give different coupling constants than would be observed with weaker magnetic fields.
E) Both A and C are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the compound below give the spin-spin splitting that would be observed for each of the protons sets in the 1H NMR spectrum. 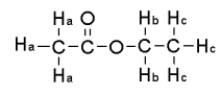
A) Ha = triplet Hb = doublet,Hc = quartet
B) Ha = singlet Hb = quartet,Hc = triplet
C) Ha = singlet Hb = pentet,Hc = quartet
D) Ha = triplet Hb = quartet,Hc = singlet
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different types of carbon would be present in the following molecule? 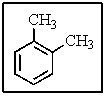
A) three
B) four
C) five
D) six
E) eight
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the 1H-NMR of the molecule shown below.Assume that JCB = 7 Hz and JCA = 1 Hz.What coupling pattern will Hc exhibit? 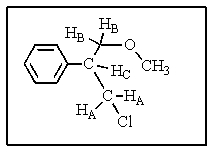
A) pentet
B) quartet
C) doublet of quartets
D) triplet of triplets
E) doublet of triplets
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetone would show how many doublets in the proton-coupled 13C NMR spectrum?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different types of hydrogens are present in the following molecule? 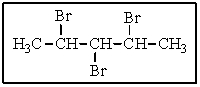
A) three
B) four
C) five
D) six
E) seven
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What structure of formula C3H6Br2 would give the following proton NMR spectrum? 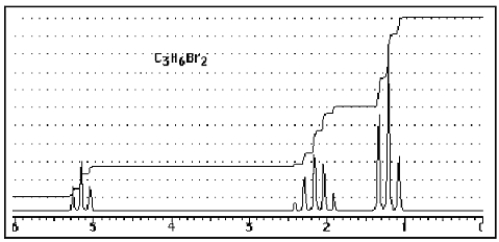
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
A seven-carbon compound which gives three signals in the 13C NMR spectrum could be:
A) heptane
B) 2-methylhexane
C) 3,3-dimethylpentane
D) 2,4-dimethylpentane
E) 2,2,3-trimethylbutane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the compound below give the integration ratio that would be observed for each of the protons sets in the 1H NMR spectrum. 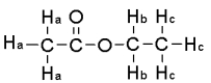
A) Ha = 3,Hb = 2,Hc = 1
B) Ha = 1 Hb = 2,Hc = 1
C) Ha = 3 Hb = 2,Hc = 3
D) Ha = 1 Hb = 1,Hc = 1
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following 1H-NMR spectrum was most likely obtained from which of the compounds listed below? 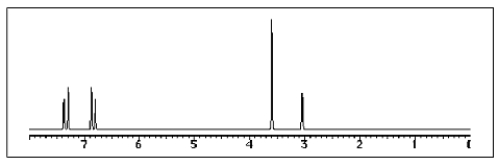
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular formulas and 13C NMR data (in ppm) are given below.The splitting pattern of each signal,taken from the non-decoupled spectrum is given in parentheses.Deduce the correct structure: 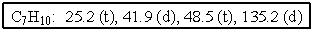
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 43
Related Exams